The Double Helix of Change: Dialectical Tensions in Evolutionary Theory
Chance, Necessity, and the Dialectics of Emergence

Table of Contents
II: Philosophical and Historical Foundations
· II.1 Precursors to Dialectical Thought in Biology
· II.2 The Rise of Dialectics in German Idealism
· II.3 Marx, Engels, and Early Evolutionary Debate
· II.4 Darwin’s Legacy
· II.5 The Modern Synthesis and the Eclipse of Dialectics
· II.6 Rediscovering Complexity: Ecology and Systems Biology
· II.7 Epigenetics, Gene Transfer, and the Reassessment of Inheritance
· II.8 Niche Construction: Organisms as Co-Creators of Environments
· II.9 Cooperation, Conflict, and the Unity of Opposites
· II.10 Philosophical Resonances and the Evolving Role of Theory
· II.11 Towards a Dialectical Evolutionary Biology
· II.12 Philosophical Foundations for Future Synthesis
III: Formal and Computational Foundations
· III.1. Mathematical Models of Feedback Loops
∘ III.1.1 Dynamical Systems as a Foundation
∘ III.1.2 Network and Graph Representations
∘ III.1.3 Memory Terms and Historical Constraints
∘ III.1.4 Assumptions, Boundary Conditions, and Validation
· III.2 Computational Modeling Techniques
∘ III.2.1 Agent-Based Models
∘ III.2.2 Evolutionary Algorithms
∘ III.2.3 Computational Complexity and Scalability
∘ III.2.4 Calibrating Simulations with Empirical Data
· III.3 Strengths & Limitations of Formal Approaches
∘ III.3.1 Capturing Dialectical Complexity
∘ III.3.2 Dealing with Non-Linearities and Emergent Phenomena
∘ III.3.3 The Role of Historical Contingency
∘ III.3.4 Risk of Oversimplification vs. Excessive Complexity
· III.4 A Convergence of Formal and Conceptual Goals
· III.5 Towards Greater Integration
IV: Integrating Key Dialectical Themes
· IV.1 Being and Becoming
· IV.2 Dialectical Natural Selection
· IV.3 Cooperation and Symbiosis
· IV.4 Horizontal Gene Transfer and Epigenetics
· IV.5 Niche Construction and Environmental Feedback
· IV.6 Chance and Necessity
· IV.7 Cross-Scale Integration
V: Empirical Validation and Testable Predictions
· V.1 Empirical Case Studies Aligned with Models
∘ V.1.1 Microbial Evolution Experiments
∘ V.1.2 Long-Term Field Studies
∘ V.1.3 Comparative Genomic and Phylogenetic Studies
· V.2. Testable Predictions and Experimental Designs
∘ V.2.1 Manipulating Feedback Loops in Controlled Ecosystems
∘ V.2.2 Multi-Generational Tracking of Epigenetic Inheritance
∘ V.2.3 Adaptive Radiations Under Changing Environments
∘ V.2.4 Measuring Dialectical Complexity in Natural Communities
· V.3 Anticipating Critiques and Refinements
· V.4 Methodological Considerations for Rigor and Transparency
· V.5 Pathways for Further Empirical Exploration
VI: Iterative Feedback & Recursive Synthesis
· VI.1 A Meta-Framework for Continuous Refinement
∘ VI.1.1 The Logic of Iterative Feedback
· VI.2 Addressing Potential Criticisms & Avoiding Circular Reasoning
∘ VI.2.1 “Dialectical Complexity” as a Vague, Catch-All Concept
∘ VI.2.2. Danger of Over-Formalization
∘ VI.2.3. Risk of Circular Reasoning
· VI.3. Strategies for Minimizing Methodological Pitfalls
∘ VI.3.1 Structured “Checkpoints”
∘ VI.3.2 Peer Collaboration and Interdisciplinary Panels
∘ VI.3.3 Encouraging Reproducibility and Data Sharing
· VI.4 The Recursive Nature of Scientific Knowledge
· VII: Conclusion and Future Directions
· VII.1 Bridging Societal and Ethical Dimensions
· VII.2 The Perspective of Dialectical Evolution

I. Introduction
Evolutionary biology has long grappled with explaining the profound diversity of life, the multitude of its forms, and the paths by which these forms have arisen, adapted, and disappeared. The Modern Synthesis, forged in the mid-twentieth century, helped consolidate classical genetics with natural selection, providing a powerful explanation for how traits change within populations under the guiding influence of selection pressures. Yet contemporary research in areas such as developmental biology, epigenetics, horizontal gene transfer, niche construction, and symbiosis has challenged the neatness of this synthesis. Consequently, many biologists and philosophers of science have sought new conceptual lenses that can better incorporate phenomena such as organism-driven environmental modification, feedback loops between phenotypes and ecosystems, and multi-level inheritance mechanisms that do not rely solely on vertical gene transmission. Against this backdrop, a dialectical perspective on evolutionary processes offers a compelling alternative — one in which adaptation, variation, and environmental context are understood as part of an ongoing, iterative interplay of forces, rather than a linear mechanism culminating in stable end-points.
By “dialectical,” one means that evolution can be viewed in terms of constant tension, negation, and synthesis of opposing elements — processes that co-define and transform one another. This resonates with classical dialectical philosophies (such as those proposed by Hegel, and later adapted by Marx) which emphasize the dynamic interplay of contradictory forces as the primary driver of change. In evolutionary biology, the “contradictions” might include phenotypic stasis versus the pressure to adapt, cooperation alongside competition, or the convergent pull of genetic inheritance mechanisms balanced against the push of ecological novelty. While mainstream evolutionary theory does acknowledge complexity, it often presents natural selection as a predominantly “negative” filter — screening out maladaptive variants — rather than as a recursive force that can transform environmental conditions, reconfigure what counts as adaptive, and shape future possibilities. In adopting a dialectical approach, we can build on existing frameworks, yet go further by explicitly modeling how an organism’s present form (being) is inseparable from its historical becoming, and how that present form, in turn, restructures the possibilities for its future evolution.
To illustrate this dialectical interplay, consider the classic example of bird wing morphology. At face value, a bird’s wing in its current manifestation reveals a remarkable fit between form and function: bones fused for lightness, feathers shaped for aerodynamic efficiency, and musculature optimized for generating lift. Traditional evolutionary narratives portray this as the outcome of millions of years of selection honing each incremental modification that conferred improved flight performance. However, a closer inspection highlights the past constraints that linger in contemporary structures — such as vestigial bones or morphological “leftovers” that were once used for other purposes — and suggests that these very constraints create the launching pad for future innovations. For instance, certain feather types, once mainly for thermoregulation or display, might later become essential for new flight maneuvers if conditions shift. In this way, the present structure (being) is never a static endpoint; it bears the imprint of historical (becoming) processes, and it prefigures possible future trajectories. This perspective, when formalized mathematically, can bring to light how historical memory or “path-dependence” modulates the adaptive landscape. Philosophically, it forces us to recognize that evolution is inherently dynamic, shaped by feedback loops between phenotypes, environments, and lineages’ ancestral legacies.
Such a dialectical viewpoint is crucial not only for evolutionary biologists but also for philosophers of science, as it bridges empirical rigor with conceptual depth. Biologists will find concrete benefits in more robust models that incorporate feedback mechanisms, non-linearities, and emergent properties — for example, gene–environment interactions that become more complex across generations. Philosophers, on the other hand, can explore how dialectical patterns in science echo or deviate from classical dialectical logic, adding nuance to longstanding debates about scientific revolutions, theoretical integration, and the interplay between chance and necessity. Indeed, one might argue that every major shift in evolutionary understanding — whether the Modern Synthesis itself or subsequent expansions — reflects a form of dialectical resolution wherein previously downplayed factors (development, epigenetics, ecological engineering) confront the existing framework and yield a new synthesis.
Despite these advantages, embracing a dialectical approach to evolution demands clear definitions and formal structures to dispel the criticism that “dialectical” language is too vague or metaphorical. Without precise formulations, dialectical accounts run the risk of degenerating into sweeping philosophical statements, disconnected from the actual data that scientists work with daily. Hence, this paper takes a two-pronged strategy. First, it examines formal mathematical and computational tools — including dynamical systems models, agent-based simulations, network analyses, and game-theoretic frameworks — to show how dialectical phenomena (e.g., environmental feedback loops, cooperative–conflict interactions, or multi-level inheritance) can be rendered in a rigorous, predictive manner. In these simple models, tension and synthesis are represented through parameter interactions, iterative updates, and emergent properties that reflect the interplay of “opposites.” Second, a narrative synthesis is developed that situates these models within the broader philosophical and historical context. By tracing how dialectical thinking emerged — albeit implicitly — in the works of Darwin, Wallace, and subsequent evolutionary theorists, one can see that a dialectical lens is not an alien imposition on biology but rather a conceptual extension of existing observations. Furthermore, modern developments like niche construction theory, horizontal gene transfer, and epigenetics can be placed into this dialectical framework, highlighting how they intensify the cyclical, feedback-driven character of evolution.
The aims, therefore, are threefold. First, the philosophical and historical underpinnings of dialectical thought in evolutionary biology are laid out, revealing a lineage of ideas that complement more conventional accounts of adaptation, fitness, and genetic inheritance. Second, an examination of formal models that ground dialectical evolution in mathematical and computational rigor. These include, for instance, differential equations that incorporate memory terms or changing fitness landscapes over time, and agent-based simulations that capture the reciprocal interactions between organisms and their self-constructed niches. In each case, an example shows how one can test the predictions of these models against empirical data — ranging from controlled microbial evolution experiments to genomic surveys of horizontally transferred genes. Third, these strands are integrated into a dialectical framework, offering a new vantage point on key evolutionary themes: being vs. becoming, cooperation vs. conflict, chance vs. necessity, and micro- vs. macro-scale processes. Rather than treating these dualisms as purely oppositional, they are illuminated as dialectical pairs whose tension and interplay fuel the evolutionary dynamic.
The intended audience spans two interlinked communities. On one hand, evolutionary biologists may be skeptical of philosophical terminologies like “dialectics” but are open to advanced modeling tools that better account for non-linear feedback and historical constraints. They may find value in the mathematical formulations and computational simulations that demonstrate how “dialectical complexity” can be measured and integrated with standard population genetics or quantitative genetics approaches. On the other hand, philosophers of science, especially those engaged in the study of the structure and evolution of scientific theories’ themselves, might appreciate how this dialectical re-casting of biological processes resonates with broader insights about contradiction, emergence, and the self-transformative character of knowledge. Where standard accounts might see the environment as a static backdrop, or treat epigenetics as an add-on to Mendelian genetics, the dialectical approach weaves these threads together, positing that the environment, the genotype, and even the scientific perspectives used to understand them co-evolve dialectically and in tandem.
Common objections from both fields are anticipted and discussed. Evolutionary biologists may wonder: “Are we merely adding superfluous philosophical jargon to well-established notions of feedback, iteration, and selection?” To counter that, substantial attention is given to testable predictions and how computational models that treat organisms as active co-creators of their niches generate different evolutionary trajectories than models assuming a passive environment. Metrics like “feedback intensity” or “dialectical complexity index” can be used in empirical studies to evaluate the degree to which new phenotypes transform and restructure selective pressures.
Likewise, philosophers might ask whether all evolutionary processes genuinely follow a dialectical logic, or whether the “dialectical” label is simply a metaphor for complexity. Here, the approach is to operationalize dialectical concepts — such as contradiction, tension, synthesis — into definable variables, emergent patterns, and explanatory loops. This operationalization allows for clearer debate about how robustly real-world evolutionary data match (or deviate from) the ideal of dialectical progression.
The central aspiration is to argue that the integration of dialectical logic and evolutionary biology is neither redundant nor merely metaphorical. Instead, it offers a rich, flexible, and empirically testable framework capable of reconciling phenomena that might otherwise appear disjointed or anomalous under prior models. From the vantage point of evolutionary biology, it furnishes new ways to conceptualize and model the interplay between organisms and their environments, highlighting that the environment is not a static setting but a dynamic participant in evolutionary processes. From the standpoint of philosophy, it invites reflection on how biological knowledge itself evolves, and how tensions within prevailing theories may give rise to more comprehensive syntheses.
In the next section, the Philosophical and Historical Foundations of the dialectical approach are explored, tracing how embryonic dialectical ideas can be glimpsed in early evolutionary thought, as well as how more recent findings (e.g., epigenetics, horizontal gene transfer) necessitate conceptual expansions. Following that, the Formal and Computational Foundations are presented, indicating how mathematical models, from simple differential equations to complex agent-based simulations, can encode feedback loops, contradictory interactions, and emergent dynamics, which are the hallmark of dialectical processes.
The heart of the paper, Integrating Key Dialectical Themes, examines specific tensions: being and becoming, dialectical natural selection, cooperation and symbiosis, horizontal inheritance, niche construction, the interplay of chance and necessity, and cross-scale integration. Each of these themes is treated as a case study in combining formal models with detailed examples — such as the bird wing’s evolutionary path, host–parasite manipulations, or cichlid fish adaptive radiations — thereby illustrating how dialectics both clarifies and is clarified by empirical data.
Subsequent sections on Empirical Validation and Testable Predictions propose ways to calibrate and test these models in microbial experiments, quantitative genomics, and real-time niche construction manipulations. Then, a reflection on Iterative Feedback and Recursive Synthesis showing how discrepancies between theory and experiment can iteratively refine both, mirroring the very dialectical processes under investigation.
If success is measured by the capacity of a framework to generate novel, empirically tractable hypotheses — and to unify rather than fragment diverse strands of data — then it is hoped that this dialectical approach can offer a fresh perspective on evolutionary theory. By examining the “contradictions” or tensions that arise at every scale of life — genetic, organismal, ecological, and macroevolutionary — one can gain nuanced insights into how these contradictions drive innovation, adaptation, and transformation across the tree of life.
II: Philosophical and Historical Foundations
Philosophical reflection on the nature of evolutionary change did not begin with Darwin. Even prior to the emergence of evolutionary biology as a formal discipline, thinkers from various traditions had already anticipated elements of what could be called a “dialectical” perspective on life and transformation. In dialectical philosophy, change arises through the interplay of conflicting forces — whereby a thesis confronts its antithesis, leading to a synthesis that transcends yet preserves aspects of both. Although such language may initially sound foreign to biology, it resonates strongly with the historical and empirical realities of how organisms adapt, compete, cooperate, and innovate.
Tracing the roots of dialectical thinking in the context of biological evolution reveals patterns of thought that connect ancient philosophical inquiries, Enlightenment-era speculations, and modern scientific debates. It also underscores why contemporary developments — such as epigenetics, horizontal gene transfer, and niche construction — might be more intelligible through a conceptual lens that treats contradiction, feedback loops, and emergent novelty as intrinsic to the evolutionary process.
II.1 Precursors to Dialectical Thought in Biology
Long before Charles Darwin formulated his theory of natural selection, there were philosophers who entertained notions of gradual change, spontaneous generation, and the transformation of species. In ancient Greek philosophy, figures like Heraclitus emphasized flux and opposition, speaking of reality as an ever-present strife of contradictory forces. While not couched in modern scientific terms, Heraclitus’s insistence on “war” as the father of all things foreshadowed later dialectical treatments of conflict and resolution. In parallel, atomists like Democritus speculated on natural processes that might give rise to living forms through aggregations of particles — a primitive version of materialistic explanations for life’s origins. Though these early ideas were far from a robust evolutionary science, they provided thematic seeds: a sense that life emerges from interplays of forces, and that change is incessant and structured by tension.
With the advent of Christianity in Europe, notions of a fixed, divinely ordered natural world became dominant, eclipsing overtly “dialectical” or materialist accounts. The medieval period largely framed biological phenomena in Aristotelian or scholastic terms, stressing unchanging essences and final causes. Nevertheless, undercurrents of inquiry persisted, such as debates about spontaneous generation or the chain of being, which sometimes gestured toward evolution-like thinking. Only in the Enlightenment did the idea of species mutability begin to resurface more explicitly, as naturalists collected empirical evidence in exploratory voyages and compiled comprehensive catalogues of flora and fauna. Yet the framework for understanding how new species might emerge, and why they might do so, remained incomplete and philosophically scattered.
II.2 The Rise of Dialectics in German Idealism
While Enlightenment thinkers charted the diversity of life, German idealist philosophers like Immanuel Kant and Georg Wilhelm Friedrich Hegel pondered how dynamic processes might unfold according to reason, contradiction, and synthesis. Kant himself was cautious about biological evolution, believing that living forms were too complex to be explained purely by mechanical laws. Hegel, on the other hand, offered a sweeping vision in which the absolute spirit unfolds through dialectical stages — each containing a conflict and resolution that paves the way for the next stage. Although Hegel did not provide a modern scientific hypothesis of organic evolution, his dialectical method highlighted that seemingly stable entities (including biological organisms) are the result of ongoing processes, replete with internal and external tensions that drive their development.
In Hegel’s view, contradiction was not merely a logical failing but a real motor of development in nature, history, and thought. Applied to the living world, this would suggest that organisms and species do not simply exist in a harmonious balance; they survive and transform through constant negotiation of competing tendencies: growth vs. decay, adaptation vs. constraint, individual vs. collective interests, and so forth. Hegel’s emphasis on negativity — where each state contains within it the seeds of its own transformation — prefigures the idea that every evolutionary adaptation carries a set of constraints that might later spur new evolutionary trajectories. Although Darwin’s theory of natural selection would soon overshadow Hegel’s more abstract speculations in the public eye, the dialectical emphasis on dynamic tension and self-transcendence would remain influential, particularly among later strands of scientific and philosophical thought.
II.3 Marx, Engels, and Early Evolutionary Debate
Karl Marx and Friedrich Engels, building on Hegel’s dialectics, turned their attention in part to the implications of Darwin’s discoveries. They found Darwin’s work on natural selection to be inspiring yet incomplete, remarking that it offered a material foundation for understanding change in nature. Engels, in particular, wrote on the dialectics of nature, attempting to show how contradictions in natural processes drive development. Though many of Engels’s specific notions — like the interplay between organism and environment or the concept of “leaps” in evolution — were more suggestive than rigorously scientific, they foreshadowed modern concerns about emergent complexity and the non-linearity of evolutionary change.
Marx and Engels also recognized that Darwin’s emphasis on competition seemed to mirror the capitalist ethos of the time, highlighting the struggle among individuals for survival. Yet they wondered whether cooperation and mutual dependence could also be viewed as dialectically intertwined aspects of life’s evolution. This line of inquiry invited later thinkers to consider that the “contradiction” between competition and cooperation might be a defining feature of living systems, with new forms of organization emerging from the dialectical interplay of these forces. Such perspectives never became mainstream in evolutionary biology, partly because Darwinian theory became strongly aligned with a more reductionist view of variation filtered by selection. However, the seeds were planted for a dialectical appreciation of how ecological and social contexts shape, and are shaped by, the traits of organisms in a recursive dance.
II.4 Darwin’s Legacy
Charles Darwin himself did not explicitly deploy dialectical language, but his notion of gradual modification through natural selection did hint at ongoing tensions — between organisms and limited resources, between varying traits within populations, and between the constraints of inheritance and the opportunities afforded by novelty. Darwin’s emphasis on incrementalism, inherited variation, and competition as a driving force resonated with the broader scientific climate of the 19th century, which sought naturalistic mechanisms for explaining complexity. Yet Darwin also stressed the influence of external conditions and recognized that “coadaptations” could be complex and interdependent. In some letters and less-cited passages of his works, he contemplated aspects of mutualism, sexual selection, and even the shaping influence of organisms on their environments. While he lacked a thorough vocabulary for describing dialectical interactions, Darwin’s writing contained hints of the recursive, multi-layered processes that would later become more explicit in theories like niche construction.
Following Darwin, figures such as Alfred Russel Wallace contributed key insights — for instance, Wallace’s distinction between natural selection operating on morphological traits and those shaping ethical or social behaviors presaged a dialectical tension between survival imperatives and emergent cultural norms in human societies. These early post-Darwinian discussions did not unify into a coherent dialectical framework, however. Instead, they scattered among various lines of inquiry: some naturalists focused on ecological interactions, others on paleontology, and still others on comparative morphology. What might have become a robust, dialectically attuned evolutionary theory instead moved in the direction of synthesizing Mendelian genetics with population-level selection models.
II.5 The Modern Synthesis and the Eclipse of Dialectics
When the Modern Synthesis crystallized in the 1930s and 1940s, uniting Mendelian inheritance with Darwinian selection, it successfully integrated the genetic basis of variation with the statistical treatment of population-level changes. This was a major triumph: it put evolutionary biology on a firmer quantitative footing, ushering in a new era of rigorous empirical testing. Yet in the process, certain nuances that might have lent themselves to dialectical interpretation became sidelined. Epigenetic variation, horizontal gene flow, and the capacity of organisms to modify their environment were seen as either marginal or as exceptions to the mainstream paradigm.
Population genetics formalized the relative frequencies of alleles under selection, mutation, migration, and drift, largely treating the environment as an external parameter rather than a co-evolving entity. While these models were powerful and elegant, their success reinforced a view of nature as a set of adaptive peaks and valleys, with selection “pushing” populations toward local optima. The inherent feedback loops — where organisms alter environments in ways that reshape the landscape of selection — remained underexplored. This omission might be seen as part of a broader intellectual climate in which linear causality and reductionist explanations were favored. Dialectics, with its emphasis on contradiction, reciprocal causation, and emergent novelty, did not fit neatly into the Modern Synthesis model of incremental adaptation. Consequently, the notion of dialectical evolution receded from mainstream consciousness, even as it survived in a few heterodox corners of biology and philosophy.
II.6 Rediscovering Complexity: Ecology and Systems Biology
In the latter half of the twentieth century, cracks in the Modern Synthesis began to appear — not invalidating it outright, but revealing unexplained complexities. Developmental biologists pointed out that genes do not act in isolation; they are embedded in gene regulatory networks that shape morphological outcomes through intricate feedback cycles. This perspective, popularized through the field of “evo-devo,” highlighted how emergent properties can arise when genes are regulated in context-dependent ways. Simultaneously, ecologists emphasized that species interact in densely connected webs, with predator–prey dynamics, mutualisms, and environmental modifications forming feedback loops at multiple scales.
Systems biology emerged as a unifying approach that recognized the importance of network interactions, non-linear dynamics, and complex emergent behaviors in living systems. These new lines of inquiry offered fertile ground for reintroducing a dialectical lens: where each node in a biological network both influences and is influenced by others, contradictions and tensions become sources of structural change. Consequently, a more relational, feedback-oriented perspective grew in popularity. Whether labeled “dialectical” or not, this shift in emphasis from single-cause linear explanations to multi-causal networks hinted at the kind of recursive logic that classical dialectical philosophy had always championed.
II.7 Epigenetics, Gene Transfer, and the Reassessment of Inheritance
A central tenet of the Modern Synthesis was that hereditary information travels almost exclusively in a vertical manner: from parent to offspring via genes. However, discoveries in epigenetics and horizontal gene transfer significantly expanded the recognized scope of inheritance. Epigenetic mechanisms, such as DNA methylation and histone modification, allow environmental factors to alter gene expression in ways that can sometimes be transmitted across generations. This introduces a feedback loop wherein an organism’s experiences can shape the developmental environment of its offspring, challenging the simplistic assumption of purely gene-driven inheritance.
Horizontal gene transfer, observed especially (though not exclusively) in bacteria, likewise disrupts the linearity of vertical descent. Genes can move laterally between unrelated organisms, sometimes enabling rapid adaptation to new environments (such as antibiotic resistance in microbial communities). In dialectical terms, these phenomena reflect contradictions within the inheritance system itself: on the one hand, there is a stable genetic lineage ensuring continuity; on the other, there are mechanisms that introduce novel genetic elements or regulatory states, potentially altering the organism–environment relationship in sudden and unpredictable ways. This tension, once thought to be marginal, has grown increasingly important in understanding microbial evolution, symbiosis, and the overall fluidity of genome architecture.
II.8 Niche Construction: Organisms as Co-Creators of Environments
Niche construction theory emerged in the late twentieth century, spearheaded by biologists like Richard Lewontin, John Odling-Smee, and others who argued that organisms do not merely adapt to pre-existing environmental conditions but actively modify them in ways that feed back into the selection pressures faced by subsequent generations. Beavers damming rivers, earthworms restructuring soil composition, and humans radically transforming landscapes are emblematic examples of how an organism can become an architect of its own adaptive landscape.
Dialectically interpreted, niche construction underscores the reciprocal cause–effect relationship between organism and environment. In a linear model, the environment “selects” among variants in a population, shaping traits via differential survival. In a dialectical model, the organism also “selects” aspects of its environment to modify, thereby reshaping future selective pressures. Contradictions arise when short-term benefits (like building a dam) generate long-term challenges (overpopulation, resource depletion) or spawn emergent opportunities (new niche availability for other species). Niche construction thus epitomizes the iterative dance of thesis and antithesis, culminating in an evolving synthesis that neither purely environment-driven nor purely organism-driven models can capture in full. Once again, the conceptual architecture to describe these interactions as dialectical is present, even if it is not always labeled as such.
II.9 Cooperation, Conflict, and the Unity of Opposites
Another crucial line of tension in evolutionary theory revolves around whether life is fundamentally competitive or cooperative. Darwin emphasized competition under scarcity, yet nature abounds with symbiotic relationships, mutualisms, and cooperative strategies — bees pollinating flowers, bacteria living symbiotically in hosts, and social insects forming sophisticated colonies. From a dialectical viewpoint, cooperation does not negate competition; rather, the two forces exist in a dynamic interplay. Cooperation can emerge from conflict, as when previously competing bacteria exchange genetic information that benefits both lineages. Conversely, cooperation can breed internal contradictions: parasites often exploit cooperative host systems, turning synergy into conflict.
Philosophical traditions have long recognized the unity of opposites, insisting that one cannot fully exist without the other. Competition can drive the formation of alliances, while cooperation can spur forms of exploitation. Modern research in evolutionary game theory, sociobiology, and microbiome studies provides empirical traction to these dialectical insights, showing how multi-level selection and overlapping interests can create layered, ever-shifting patterns of conflict and collaboration. This perspective opens new questions about how complex communities, from coral reefs to gut flora, evolve over time through negotiated trade-offs — precisely the sort of phenomenon a dialectical framework can elucidate.
II.10 Philosophical Resonances and the Evolving Role of Theory
Philosophers of science have a deep interest in how theories evolve in response to anomalies, contradictions, and conceptual tensions. When viewed historically, scientific revolutions — like the shift from a static, creationist worldview to Darwinian evolution — often reflect dialectical surges of new insights that challenge established paradigms. The resolution of these tensions forms a new synthesis, which itself harbors latent contradictions that eventually lead to further transformation. In evolutionary biology, we see this pattern with the Modern Synthesis: it reconciled Mendel’s genetics and Darwin’s natural selection but left out developmental plasticity, epigenetics, and active environment-modification by organisms. Over the last few decades, these neglected factors have resurfaced with increasing force, suggesting another potential paradigm shift — one that might be captured by an explicitly dialectical view of nature and inheritance.
Philosophers analyzing the structure of scientific theories can find in evolutionary biology a microcosm of dialectical progression. Each time a new phenomenon — be it horizontal gene transfer or niche construction — pushes the boundaries of the prevailing model, the discipline either integrates the phenomenon into its existing framework or resists it until compelling data demand acceptance. In principle, a robust dialectical methodology anticipates such tensions and does not require ad hoc expansions; it welcomes contradictions as engines of theoretical advancement. Thus, evolutionary biology could benefit from a meta-theoretical stance that is intrinsically open to self-transformation, rather than presenting a closed system with narrowly defined parameters.
II.11 Towards a Dialectical Evolutionary Biology
Many of the threads mentioned above — epigenetic inheritance, niche construction, and multi-level interactions — have gained traction independently, spawning subfields and specialized research programs. Yet they often remain fragmented, lacking a unifying perspective that treats reciprocal causation and emergent properties as core principles. Dialectics, with its emphasis on the interplay of opposites, offers precisely such an integrative vision. It shows that inheritance, adaptation, and environment are interdependent, each both shaping and being shaped by the others in continuous loops of transformation.
This perspective illuminates why empirical anomalies once dismissed as marginal might be central to understanding life’s complexity. A dialectical approach does not dismiss simpler, reductionist models; it recognizes their power within certain domains while pointing to the limitations they face once feedback loops become prominent. It also highlights that different scales of biological organization — genes, cells, organisms, ecosystems — cannot be fully explained in isolation, since changes at one level often trigger responses at another. The repeated pattern is tension, negotiation, and the emergence of new organizational levels or processes, mirroring the dialectical triad of thesis, antithesis, and synthesis.
II.12 Philosophical Foundations for Future Synthesis
The philosophical ramifications of incorporating a dialectical approach in evolutionary biology are significant. On an epistemological level, it entails accepting that knowledge of evolutionary processes may never be final or absolute, because each discovery reshapes the conceptual boundaries within which it is interpreted. Contradictions in data — such as the discovery of new inheritance systems or surprising examples of cooperation — are not mere aberrations but catalysts for conceptual innovation. Methodologically, this calls for transdisciplinary collaborations: modelers and empirical researchers working alongside philosophers who can articulate the conceptual frameworks needed to integrate disparate lines of evidence. This synergy, in turn, can shape how research questions are formulated, how experiments are designed, and how scientific explanations are structured.
Dialectics thus invites biologists to see their theories as evolving, historically contingent constructs — open to revision as new contradictions arise. Rather than viewing competing or seemingly incompatible findings as obstacles, it positions them as opportunities for synthesis. In practice, this outlook can generate fresh hypotheses: for example, that epigenetic mechanisms do more than store genealogical memory — they might also create new forms of evolutionary “contradiction” by enabling rapid reversals or expansions of phenotypic states. Or that symbiotic relationships should be understood as sustained negotiations involving conflict and cooperation, which periodically reconfigure the boundaries between “host” and “symbiont.” Rather than resolving such tensions once and for all, dialectical thinking shows how they persist, fueling ongoing transformation in the biological realm.
The next steps in forging a dialectical evolutionary biology involve moving beyond historical and philosophical insights toward rigorous and testable models — an enterprise that will demand computational simulations, quantitative metrics, and carefully designed experiments. As the subsequent sections will show, these endeavors can draw inspiration from dialectical philosophy’s emphasis on interactive complexity, offering powerful frameworks for capturing how life evolves through ongoing cycles of constraint, innovation, and transformation. By situating these developments within their deeper philosophical lineage, we enrich our understanding of evolution as more than a mere scientific theory: it becomes a testament to how contradictions and tensions shape the living tapestry of the natural world and, in turn, our evolving comprehension of it.
III: Formal and Computational Foundations
A central challenge in applying a dialectical framework to evolutionary theory lies in giving precise, testable form to the concepts of feedback, contradiction, and emergent novelty. While philosophical reasoning sets the stage for seeing evolution as a web of interlocking tensions, mathematical and computational models translate those insights into explicit structures that can be analyzed, simulated, and tested against empirical data. This section details two major avenues for formalizing dialectical processes in evolution: first, the use of mathematical models of feedback loops — often drawing from dynamical systems theory and network analysis — and second, the development of computational simulations in the form of agent-based models, evolutionary algorithms, and related numerical methods. Across these approaches, the guiding principle is to capture how organisms, traits, and environments interact in recursive ways, thereby illuminating the iterative and historical character of evolution.
III.1. Mathematical Models of Feedback Loops
III.1.1 Dynamical Systems as a Foundation
Many phenomena in evolutionary biology are conveniently modeled using dynamical systems, which describe how a set of variables changes continuously over time. In the simplest scenario, one might consider a population characterized by one or more phenotypic traits x(t). A differential equation of the form
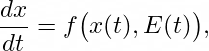
where E(t) represents the environment, encapsulates how the trait’s rate of change depends both on its current value and on environmental factors. In a standard, non-dialectical approach, the environment E(t) is often treated as an external parameter, possibly constant or periodically varying. However, a dialectical view asserts that E(t) itself should be influenced by x(t), creating a feedback loop:
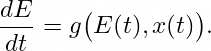
Now, the organism’s traits and the environment co-evolve in tandem. g(⋅) is the rate of environmental change function, specifying how fast and in what manner the environment shifts in response to both its own current state and the organism’s traits or actions. If x(t) grows larger — say it represents a trait that extracts more resources — this might deplete or transform the environment, subsequently altering the selection pressure on the trait itself. This coupled system can exhibit non-trivial dynamics, such as the emergence of oscillations, multi-stable equilibria, or chaotic regimes, all of which reflect dialectical tensions in which each component pushes back on the other.
To illustrate the importance of feedback loops, consider a trait related to resource consumption, for example, the efficiency of foraging in a population of microorganisms. As efficiency increases, individuals might consume resources more rapidly, leading to resource depletion. Resource depletion in turn imposes a selection pressure on further efficiency gains — possibly up to a point where it becomes too costly to maintain those adaptations. Beyond that point, the environment may collapse if overexploited. In purely linear or one-way models (where the environment is fixed), the analysis might suggest a stable equilibrium of moderately efficient foragers. However, once feedback is included, the system might experience recurring cycles — populations overexploit resources, crash, partially recover, and so forth — mirroring a dialectical interplay of adaptation and self-imposed constraint.
III.1.2 Network and Graph Representations
While coupled differential equations are a powerful tool, many evolutionary phenomena involve discrete interactions or multiple interacting traits, making network models a natural choice. In a network representation, each node might correspond to a trait, genotype, or ecological factor, and edges encode influence relations or flows of material and information. For instance, a genotype network can describe how different alleles or phenotypic states connect to one another through mutation or recombination pathways. A dialectical element emerges when certain transitions feed back to change the connectivity or weights of the network itself — for example, the appearance of a new beneficial trait that alters not only the fitness of related genotypes but also the rates of migration or resource exchange across the network.
One can formalize these ideas by assigning dynamic weights to the edges, letting them fluctuate as a result of changes within nodes (traits, organism counts, or resource levels). Suppose Wxy(t) is the weight of an edge between nodes x and y at time t. If node x represents a phenotype that modifies environmental conditions, it might increase or decrease the capacity of node y (another phenotype or an environmental variable) to thrive. This causes Wxy(t) to evolve. Because each node’s state also depends on incoming edges, the dialectical nature emerges as the entire network reconfigures itself over time based on interactions shaped by historical events.
III.1.3 Memory Terms and Historical Constraints
A hallmark of dialectical thinking is that the present is always shaped by the past. In formal terms, this can be captured by introducing memory or delay terms into equations. Instead of having instantaneous rates of change, a system might evolve according to:
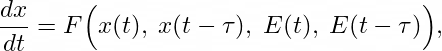
where τ is a time delay capturing how past states continue to exert influence. Such memory terms are crucial for modeling processes in which historical conditions leave lasting imprints — like epigenetic modifications that persist across cell divisions, or the lingering consequences of niche construction. This approach can lead to rich dynamical behavior, including multi-armed oscillations and sudden regime shifts triggered by events that occurred in the system’s past. By incorporating memory, the model resonates with the dialectical idea that an organism’s “being” encapsulates its “becoming,” i.e., that current phenotypic states reflect accumulated historical constraints and innovations.
III.1.4 Assumptions, Boundary Conditions, and Validation
Mathematical models inevitably rely on simplifying assumptions. For a dialectical system, these assumptions often revolve around:
- Form of Feedback: Is it direct (e.g., one variable instantly affects another) or mediated (e.g., requiring accumulation of resources or signals)?
- Linear vs. Non-linear: Many real-world dialectical interactions are non-linear, making analytical solutions challenging but capturing emergent phenomena more accurately.
- Boundary Conditions: Are variables constrained (e.g., resource levels cannot drop below zero), and do boundary conditions shift over time (e.g., seasonal changes or migrations)?
Validation of such models typically involves parameterization with empirical data — perhaps from laboratory evolution experiments where resource inputs, population sizes, and trait distributions are measured across generations. Modelers compare predicted trajectories (e.g., population booms and busts) to observed data, adjusting parameters to see if the feedback loop structure genuinely accounts for the patterns. Sensitivity analysis is also pivotal: if small changes in initial conditions or parameter values produce drastically different outcomes, the system might exhibit chaotic or near-chaotic dynamics, underscoring how historical accidents can shape evolutionary paths.
III.2 Computational Modeling Techniques
III.2.1 Agent-Based Models
While differential equations and network analyses often assume well-mixed populations or continuous changes, real evolutionary processes can hinge on the discrete actions of individual organisms or local interactions. Agent-based models (ABMs) tackle this challenge by simulating many “agents” (representing organisms, cells, or genes) that operate under defined rules in a shared environment. Dialectical processes emerge as these agents modify the environment, which in turn constrains or enables new actions.
- Local Interactions: In ABMs, organisms are typically located on a lattice, graph, or continuous spatial field. Their movement or resource consumption affects their immediate neighborhood, creating local resource depletion or enrichment.
- Adaptive Behavior: Agents can alter their strategies or phenotypic traits in response to local conditions — mimicking how selection might favor certain behaviors under constraints that themselves evolve.
- Adaptive Landscapes: As each agent adapts, the collective behavior can reshape the “fitness landscape,” making it a moving target. Consequently, there is no single equilibrium but rather a fluid process of co-evolution.
This approach naturally encodes dialectical tension: for instance, a cluster of highly efficient resource exploiters might degrade local resource patches to such an extent that they must either migrate or risk extinction, perhaps giving an advantage to less efficient but more frugal agents in the long run. ABMs can produce complex spatiotemporal patterns — fronts, waves, and patchy ecosystems — that are impossible or impractical to capture using homogeneous population models. They thus serve as “virtual laboratories” where emergent phenomena — cooperation, competition, environmental engineering, and even cultural traits — can be studied in detail.
III.2.2 Evolutionary Algorithms
In computational fields, evolutionary algorithms (EAs) — which include genetic algorithms, evolutionary strategies, and genetic programming — were inspired by biological evolution. They maintain a population of candidate solutions (analogous to organisms) which are subjected to variation (mutation, recombination) and selection. Typically, the “environment” is encoded as an objective function that ranks the fitness of each candidate.
To make EAs more dialectical, one can introduce dynamic fitness landscapes that co-evolve with the population, or incorporate multi-level selection where both individuals and groups undergo selection. For example, a multi-level evolutionary algorithm might treat each subpopulation as an evolving entity in its own right, while the entire system is also selected based on collective properties. Another approach is to allow solutions to rewrite or modify the rules of the environment as they progress — mimicking niche construction or ecological engineering. This can generate significantly different evolutionary trajectories than static EAs, producing more diverse, and sometimes more creative, solutions.
Researchers sometimes embed epigenetic or horizontal gene transfer analogies in EAs, permitting the direct transfer of partial solution structures between individuals outside of standard recombination protocols, or toggling “on” and “off” certain traits based on environmental triggers. The resulting models can be tested on real-world optimization problems or computational tasks, providing indirect but intriguing insights into how dialectical interactions might shape innovation, stability, and adaptability in natural systems.
III.2.3 Computational Complexity and Scalability
One of the main challenges in agent-based and multi-level evolutionary simulations is computational complexity. As the number of agents grows or the environment’s detail increases, the number of possible interactions explodes, quickly taxing available computational resources. Moreover, dialectical feedback loops can produce highly non-linear effects where small initial differences in agent states or spatial distributions lead to drastically different outcomes. This sensitivity to initial conditions reflects a hallmark of dialectical processes — historical contingencies matter. But it also means that extensive replications and parameter sweeps are necessary to robustly characterize model behavior.
To address these challenges, researchers often adopt high-performance computing techniques, parallelizing agent-based simulations across multiple processors or using specialized algorithms for scheduling interactions. GPU-based acceleration can speed up computations involving large numbers of agents performing similar operations. In large-scale ecological or evolutionary models, hierarchical or multi-resolution approaches may be employed: for instance, an ABM at the local scale that periodically informs a coarser model at the regional scale, ensuring that fine-grained interactions remain tractable while capturing broad feedback effects.
III.2.4 Calibrating Simulations with Empirical Data
A key step in formalizing dialectical concepts is empirical calibration. While ABMs and EAs can generate fascinating behaviors in silico, their relevance hinges on how well their assumptions match real biological processes. For microbial or short-lived organisms, laboratory experiments offer an avenue to measure trait distributions, resource consumption rates, mutation rates, and environmental changes. These measurements can be used to initialize agent populations or to parametrize rules (e.g., “if local resource X < threshold, shift from a cooperative to a selfish strategy”).
One illustrative example involves microbial consortia that engineer their environment by excreting metabolic byproducts. Researchers can track pH, nutrient gradients, or toxin concentrations over time in well-controlled microcosms. An ABM can then be set up where each agent’s metabolic rules approximate the observed real-world metabolism, and the shared medium’s composition updates in each time step. By adjusting these rules to fit real data, scientists can test whether the model accurately reproduces spatiotemporal patterns (e.g., wave-like expansions, layering of species, or cyclical resource usage). If it does, confidence in the underlying dialectical representation grows. If not, researchers refine the feedback rules or incorporate new empirical insights about genetic regulation, mutualistic interactions, or emergent behaviors.
III.3 Strengths & Limitations of Formal Approaches
III.3.1 Capturing Dialectical Complexity
A core strength of these formal approaches — dynamical systems, network models, ABMs, and evolutionary algorithms — is their flexibility in encoding feedback loops, memory effects, and multi-level interactions. This ability to represent dialectical complexity stands in sharp contrast to simpler, linear models that treat the environment as constant or external. Through carefully chosen parameters and structures, it becomes feasible to explore how contradictions (e.g., between resource use and resource renewal) drive emergent patterns and how innovations feed back to reshape the adaptive landscape.
Moreover, these models make it possible to operationalize concepts like “dialectical tension” by quantifying rates of change, measuring synergy indices in cooperative interactions, or tracking how quickly environmental alterations feed back to trait evolution. Researchers can thus move beyond metaphor to identify statistical signatures (e.g., cyclical dynamics, abrupt phase transitions, localized clusters of specialization) that indicate strong feedback processes at work.
III.3.2 Dealing with Non-Linearities and Emergent Phenomena
Non-linearities are a double-edged sword. On one hand, they are essential for capturing how small changes in conditions can lead to large-scale evolutionary shifts, or how contradictory pressures can trigger transitions in population structure. On the other hand, non-linear systems can defy intuitive analysis, often lacking closed-form solutions. Dynamical systems might exhibit chaotic regimes where long-term predictions are impossible, or they might settle into stable attractors that hide the underlying dynamics. ABMs, likewise, can generate emergent behaviors not easily deducible from their local rules.
To handle these complexities, modelers employ bifurcation analysis, stability analysis, and advanced visualization tools (e.g., phase portraits, state-space trajectories, or time series plots). These techniques can reveal whether the system transitions from one qualitative state to another as parameters cross certain thresholds — mirroring a dialectical “negation” that yields new states (or “syntheses”). In ABMs, researchers often track summary statistics like cluster size distributions, average phenotypic diversity, or resource patchiness to detect emergent structures. Identifying these structures can highlight how the system’s internal contradictions generate self-organizing behavior.
III.3.3 The Role of Historical Contingency
Because a dialectical approach emphasizes historical contingency, formal models that incorporate path dependence, memory, or time-lagged feedback are especially pertinent. Yet there is a risk of overfitting or generating a myriad of free parameters — each capturing a distinct historical element. Balancing specificity with generality is thus crucial. Ideally, a dialectical model should:
- Specify the essential feedback loops and memory components that shape evolution.
- Remain tractable enough to allow systematic parameter exploration.
- Incorporate real data where available, or at least produce empirically falsifiable predictions (e.g., “in the presence of a certain resource dynamics, a cyclical population crash–recovery pattern should emerge after about N generations”).
Without empirical anchoring, historical “baggage” in models can become an abstract flourish rather than a meaningful mechanism. Consequently, modelers often rely on well-characterized experimental systems — like microbes evolving in chemostats or viruses in repeated bottleneck passages — to ground their historical variables in measurable phenomena, such as time-lagged morphological changes or gene expression states.
III.3.4 Risk of Oversimplification vs. Excessive Complexity
Models can fail in two opposite ways: oversimplification (failing to include important dialectical feedbacks) or excessive complexity (becoming so parameter-rich and context-specific that they lose explanatory clarity). Threading the needle requires iterative refinements and a readiness to prune or reconfigure model components that do not meaningfully contribute to predictive power. This iterative approach — where a model is built, tested, refined, and re-tested — reflects the dialectical principle that knowledge itself evolves through confrontation with data and theoretical tensions.
III.4 A Convergence of Formal and Conceptual Goals
Formal and computational models bring rigor and testability to what might otherwise be abstract dialectical insights. They also help unify phenomena that can seem disparate: from genotype-phenotype mapping to ecological interactions and macroevolutionary patterns. By specifying how organisms and environments co-evolve, how contradictions fuel transitions in population structure, and how historical legacies inform present dynamics, these models function as “miniature worlds” in which dialectical evolution unfolds.
Concomitantly, the conceptual impetus from dialectical thinking broadens the realm of modeling. Rather than focusing on equilibrium or single “optimal” solutions, one is drawn to exploring dynamical landscapes with multiple attractors or long transient behaviors — i.e., systems that do not simply settle but keep shifting as new tensions surface. Thus, the spirit of dialectics encourages exploring:
- Oscillatory or punctuated dynamics: Systems that alternate between expansions and contractions, or remain stable for long periods before radical shifts.
- Phase transitions triggered by synergy: Where new cooperative or structural features appear once a critical threshold is exceeded.
- Historical–context dependence: Where the same set of parameters yields different outcomes depending on the sequence of preceding events.
In practice, applying these formal methods to concrete biological systems will typically require iterative collaboration among theoretical biologists, computational scientists, and empirical researchers. The process might unfold as follows:
- Identify a Key Dialectical Interaction: For instance, the feedback loop between a trait related to resource use and the resource itself.
- Choose an Appropriate Formalism: A continuous dynamical model if changes are gradual, a discrete ABM if local interactions or spatial structure is pivotal.
- Incorporate Historical/Memory Elements: Decide which aspects of the past (e.g., epigenetic states, morphological constraints, or niche modifications) significantly alter present dynamics.
- Parameterize and Validate: Gather empirical data (growth rates, resource depletion curves, trait frequencies) to calibrate. Run simulations or solve equations, compare with observed time-series or patterns.
- Refine: Adjust the model’s rules or parameters based on discrepancies, possibly introducing new dialectical tensions or removing unneeded complexities.
This cyclical modeling approach exemplifies how the methodology of building and testing a dialectical model itself becomes a dialectical process. Each mismatch between model predictions and empirical reality acts like a “contradiction,” prompting further refinement or rethinking of the model’s assumptions.
III.5 Towards Greater Integration
Formalizing dialectical evolution is still a nascent endeavor. As computing power expands and scientists gain access to finer-scale genomic and ecological data, the possibility of capturing multi-level, feedback-rich processes in a cohesive model becomes increasingly realistic. Ongoing developments include:
- Multi-Scale Modeling: Combining gene regulatory networks (modeled via discrete or continuous dynamical systems) with ABMs of organism-level interactions and macroscale ecological or evolutionary processes.
- Hybrid Approaches: Linking deterministic frameworks (e.g., partial differential equations for resource diffusion) with agent-based compartments that handle discrete events (e.g., organism births, resource depletion).
- Incorporating Epigenetics and HGT: Programming rules in ABMs or EAs that allow for horizontal transfer of traits or environmentally induced epigenetic switches, capturing even more nuanced dialectical loops.
- Data-Driven Techniques: Employing machine learning or Bayesian inference to detect signatures of feedback-driven or dialectical dynamics directly from large biological datasets — gene expression time-series, population genomic data, or ecosystem-level measurements.
The ultimate goal is not to create a monolithic “Theory of Everything” but rather to demonstrate how dialectical logic can guide the design of models that better reflect the recursive and co-creative aspects of evolution. By formalizing these ideas, scientists gain access to quantitative predictions and the potential to run in silico experiments that would be impractical or impossible in real ecosystems. Such explorations can generate new hypotheses about how contradictions in resource use, or emergent forms of cooperation, might appear under specific parameter regimes or historical conditions — hypotheses that can then be tested in controlled lab settings or field studies.
This section has outlined the conceptual and methodological tools by which one can rigorously embed a dialectical perspective into evolutionary models. Each technique contributes a unique vantage on how feedback loops and interacting opposites structure the evolutionary process. The non-linear and dynamic nature of these models resonates with the core dialectical principle that no component — organism, trait, or environment — remains static when placed in mutual tension with the others.
Building on these foundations, subsequent sections wll integrate them more explicitly with empirical case studies — spanning cooperation, niche construction, horizontal gene transfer, and cross-scale interactions — thereby demonstrating how a dialectical approach can guide both scientific inquiry and conceptual innovation in evolutionary biology.
IV: Integrating Key Dialectical Themes
Formally modeling dialectical processes in evolution provides a robust scaffold, but these concepts gain their fullest expression when applied to concrete biological themes that exemplify ongoing tensions and recursive feedbacks. This section explores seven core themes — Being and Becoming, Dialectical Natural Selection, Cooperation and Symbiosis, Horizontal Gene Transfer and Epigenetics, Niche Construction and Environmental Feedback, Chance and Necessity, and Cross-Scale Integration — each offering a unique vantage on how oppositional or complementary forces can intertwine to drive evolutionary change. In every case, the dialectical framework reveals patterns and complexities that more linear or reductionist approaches often overlook.
IV.1 Being and Becoming
In a dialectical view, an organism’s “being” (its present form and function) is the crystallization of its historical “becoming” — the sequential accumulation of genetic, developmental, and ecological legacies. Simultaneously, this present state serves as the launching pad for new evolutionary possibilities. The tension lies in the fact that past adaptations can either constrain future change (e.g., morphological “lock-ins” that reduce flexibility) or enable it (e.g., exaptations that open up new functional roles). Formally, one might encode this interplay as a delay differential equation system where current trait values not only depend on instantaneous conditions but also retain “memory” terms from earlier developmental or evolutionary states.
An illustrative case arises in avian wing morphology. Birds carry vestiges of ancestral limb structures — fused bones, specialized feather arrangements — that both reflect deep evolutionary history and shape the aerodynamic possibilities of the future. Birds with certain wing-loading ratios might be highly optimized for gliding but limited in take-off dynamics, illustrating how morphological “lock-ins” can stifle specific innovations. Conversely, the morphological basis for flight feathers once evolved for thermoregulation can enable entirely new flight styles, underscoring how “being” sets the stage for “becoming.”
To capture such historical imprinting, one might define a trait vector x(t) representing wing-related attributes (e.g., skeletal mass, feather microstructure). A delay term x(t−τ) can influence x(t) through parameters that reflect mechanical or developmental inertia. In a population-level model, selection coefficients might be functions of both current environment E(t) and historical states E(t−τ). If historical conditions strongly favor a certain morphological arrangement, the population could become “locked” in that region of the trait space, highlighting a path-dependent or hysteresis effect.
A fair criticism is that focusing on “historical constraints” can be too vague. However, dialectical modeling responds by introducing explicit quantitative metrics — say, the path dependence index (PDI), which measures how divergence from a baseline trait trajectory affects present fitness outcomes. The higher the PDI, the stronger the historical constraint. Empirical data (e.g., fossil records, morphological series) can be used to calibrate the timeline τ and to confirm whether the system truly exhibits memory-induced dynamics.
IV.2 Dialectical Natural Selection
Standard accounts of natural selection often cast it as a unidirectional filter: beneficial mutations spread, deleterious ones are removed. From a dialectical standpoint, selection becomes an iterative, reciprocal force that shapes and is reshaped by the evolving traits themselves. In other words, once novel adaptations appear, they can reconfigure the adaptive landscape for subsequent variations. This cyclical pattern is well exemplified by adaptive radiations, where an initial breakthrough (e.g., specialized beak shape) changes ecological opportunities, which in turn invites new trait diversifications.
Take Darwin’s finches in the Galápagos Islands. The classical story involves variation in beak morphology leading to differential survival based on seed type. Yet a dialectical extension observes that as certain beak forms proliferate, seed availability and competition patterns shift. A “dominant” beak form might deplete its favored seed type more rapidly, opening a niche for alternative forms. Here, selection is not just “negative elimination” but a dynamic that recurrently transforms the environment and the fitness payoffs for other lineages.
One can model dialetical natural selection using a network or graph-based approach where nodes are trait configurations (e.g., beak shapes), and edges represent possible evolutionary transitions (mutational paths). Fitness at each node depends on both historical usage of resources (which modifies resource distribution) and the presence of other co-existing nodes. This yields an adaptive network: traits and environment co-evolve, causing changes in edge weights or node fitness as certain configurations become prevalent. Over time, stable sub-networks may emerge — only to be destabilized once an innovation unexpectedly opens new resource channels.
One might argue that standard population genetics already includes frequency-dependent selection, so labeling it “dialectical” is superfluous. The difference is that dialectical models emphasize the deep entwinement of historical contingency, feedback loops, and emergent novelty, rather than simply adjusting selection coefficients in real time. They invite explicit tracking of feedback intensity — a parameter measuring how strongly an existing trait modifies the environment — and propose testable predictions about cycles of innovation and stasis.
IV.3 Cooperation and Symbiosis
Evolutionary theory has often toggled between emphasizing competition (in line with the “struggle for existence”) and recognizing the widespread prevalence of cooperative or symbiotic relationships. Dialectically speaking, cooperation and conflict constitute a pair of opposites that can generate novel adaptive structures through their dynamic interplay. Symbiotic relationships can vacillate between parasitism and mutualism, each tension provoking genetic, behavioral, or ecological adjustments.
A compelling instance arises in coral–algal symbioses, where reef-building corals host photosynthetic algae. The algae supply corals with nutrients derived from photosynthesis, while corals offer shelter. However, under stress (e.g., temperature rises), the relationship can degrade into coral bleaching, akin to a breakdown in cooperation. From a dialectical perspective, each breakdown can spur selective pressures favoring strains of algae more tolerant of heat or corals that can either switch symbionts or survive without them. Over multiple generations, a dynamic cycle emerges — cooperative synergy breeding dependence, and environmental shocks reintroducing conflict that fosters new pathways toward (re)cooperation or alternative survival strategies.
In a game-theoretic model, each partner’s payoff depends on the other’s strategy — cooperative vs. exploitative. A dialectical extension would allow for strategy switching to evolve if environmental feedback changes the payoff matrix. For corals and algae, the payoff might shift drastically with temperature stress or nutrient availability, meaning that the stable equilibrium under normal conditions becomes unstable under stress, driving new strategies. This yields a phase transition in the system’s evolutionary dynamics, reflecting the contradictory pulls of synergy (cooperative advantage) and conflict (evolutionary “cheating” or exploitation).
Critics might say that applying a “dialectical lens” to cooperation vs. conflict is conceptually interesting but lacks empirical specificity. Yet one can quantitatively analyze, for example, the proportion of time or the fraction of individuals in cooperative vs. exploitative states across environmental gradients. Experimental microcosms (like bacterial communities engaged in cross-feeding) can measure real-time transitions between stable cooperative interactions and emergent conflict, testing predictions about how changing resource contexts or new mutants might tip the balance.
IV.4 Horizontal Gene Transfer and Epigenetics
Traditional evolutionary models assume that genetic information flows vertically — parent to offspring — so changes accumulate incrementally. However, phenomena like horizontal gene transfer (HGT) and epigenetic modifications inject new forms of inheritance that can drastically alter evolutionary trajectories. In dialectical terms, they introduce contradictions between stable lineage identity and the infiltration of novel genetic or regulatory elements. This tension can jump-start new adaptations or disrupt established equilibria, underscoring that inheritance is not a simple linear channel but a dynamic process shaped by extrinsic and intrinsic factors.
The rise of antibiotic resistance among bacteria offers a striking demonstration. Resistance genes spread laterally across species via plasmids or transposons, effectively rewriting the microbial “fitness landscape” overnight. Epigenetic phenomena likewise shape adaptation in higher organisms, with stress-induced methylation patterns persisting across generations, thus altering developmental pathways. Both processes show that an organism’s evolutionary potential can shift abruptly, not necessarily in step with standard mutation–selection models.
One might use network diffusion models where nodes represent genotypes or epigenetic states, and edges allow for lateral transfer with certain probabilities. Incorporating feedback means that the success of a horizontally transferred gene depends on the current genomic or epigenetic context, which may itself shift as new genes arrive. For epigenetics, a two-tier model can track both “hard-coded” genomic changes and “soft-coded” modifications in parallel, with the latter responding more swiftly to environmental stimuli. This layered structure captures how ephemeral epigenetic states can become, under certain conditions, stabilized into new genetic or epigenetic equilibria — precisely the kind of emergent dynamic that a dialectical viewpoint highlights.
Skeptics may dismiss lateral gene transfer or epigenetic inheritance as side phenomena irrelevant to core evolutionary patterns. Dialectical modeling pushes back, pointing to the contingent yet potentially revolutionary nature of these events. Empirical data from microbial communities, plant stress responses, or insect polyphenisms can quantify the rate and impact of non-vertical inheritance on morphological or behavioral innovation. The question is not whether these mechanisms are always at play, but whether they can transform evolutionary outcomes in crucial junctures, thus embodying the dialectical principle of contradiction instigating new syntheses.
IV.5 Niche Construction and Environmental Feedback
Niche construction theory posits that organisms are not mere passive recipients of selection but active co-creators of their ecological niches. By building dams, digging burrows, or excreting metabolic byproducts, they alter environmental conditions that then loop back to shape subsequent adaptation. Dialectically, niche construction underscores that each organism–environment relationship is a two-way street, replete with feedback loops that can accelerate or redirect evolutionary change.
The beaver is an oft-cited example: By constructing dams, beavers create ponds and wetland habitats that not only favor beaver survival but also affect fish populations, local vegetation, and soil nutrient dynamics. Over time, these environmental transformations impose new selective pressures on beaver physiology and behavior, potentially favoring improved wood-gnawing efficiency or particular social structures. Similar logic applies to microbial mats in which secreted extracellular polymers form new microhabitats, altering pH or oxygen gradients and reshuffling the selective environment within mere hours or days.
Time-lagged differential equations or iterative map models can reflect how each incremental environmental change feeds back on population growth or trait development. For instance, let E(t) represent water flow or vegetation density, and let x(t) be the population or a suite of traits in the beaver. If x(t) crosses a threshold — for instance, a critical population size needed to construct large dams — E(t) may shift drastically (e.g., river flow is reduced, leading to a stable pond). That new environmental state then exerts selection for further improvements in dam-building, forming a self-reinforcing loop. Over many generations, this can produce ecological “hotspots” of evolutionary diversification in and around the constructed niche.
One criticism is that niche construction can be “just another factor” in selection, not warranting a special category. Dialectical modeling clarifies that what sets niche construction apart is the internal feedback it introduces; the environment is no longer exogenous but evolves as an endogenously coupled system. Empirical tests might measure shifts in local biodiversity or resource distribution before and after niche construction events to see if predictions of cyclical or phase-transition dynamics hold.
IV.6 Chance and Necessity
Ever since Monod’s famous formulation of “chance and necessity,” evolutionary theorists have wrestled with how to integrate random mutations (chance) and deterministic selection (necessity) into a unified perspective. Dialectics reframes this duality as a productive tension: chance events can open new evolutionary pathways, while necessity sculpts the viability of these paths. At times, random accidents (e.g., population bottlenecks, founder effects) trigger substantial lineage reorganizations that then converge on new stable phenotypic forms.
Viruses evolving in repeated bottleneck passages — such as when a small number of virions infect a new host — epitomize how chance can drastically alter which variants survive, while the necessity of host immune pressures shapes the subsequent fixation of those variants. Over many cycles, the interplay of random drift in population bottlenecks and strong directional selection for immune evasion can produce novel phenotypes not predictable from an initial genotype or environment alone.
One way to formalize chance and necessity dialectically is via stochastic differential equations that include deterministic selection terms and random drift or mutation terms. The environment might itself shift unpredictably, sometimes amplifying the impact of a rare beneficial mutation or, conversely, annihilating a promising lineage via a random stress event. By simulating the system across multiple replicate runs, one observes distributions of possible outcomes, revealing stable attractors favored by necessity and branching pathways seeded by chance. Such models predict that certain “lucky” lineages can achieve rapid expansions if they align with fleeting windows of ecological opportunity — again underscoring the tension between random impetus and deterministic sculpting.
A common objection is that standard population genetics already accommodates chance (genetic drift) and necessity (selection). The dialectical distinction lies in explicitly modeling how random events can lead to qualitative reconfigurations of the adaptive landscape itself. One can measure the “landscape plasticity” that emerges as lineages shift trait–environment configurations, turning improbable occurrences into catalysts for radical transformations. Empirical tests might involve repeated evolution experiments under identical conditions: if the outcomes diverge widely, that signals a high synergy between chance and environmental feedback.
IV.7 Cross-Scale Integration
Evolution unfolds across multiple hierarchical levels: genes, cells, organisms, populations, communities, and ecosystems. Each level can impose constraints or provide opportunities for the others. A dialectical approach insists that these scales are mutually entangled, with emergent macro-level structures (e.g., new ecosystem forms) feeding back on micro-level processes (e.g., gene regulation). The tension arises when the conventional partitioning of scales overlooks how small-scale events might cascade upward — or how large-scale conditions might regulate or select among micro-scale variations.
Consider multicellularity as an emergent property. Individual cells, initially in loose associations, face contradictions between cooperating for mutual benefit and competing for resource uptake within a cluster. Over evolutionary time, these tensions can yield genetically encoded pathways for coordinated growth, specialized cell types, and group-level homeostasis — transformations that, in turn, feed back on the gene-level dynamics (e.g., differential expression of adhesion proteins). At the macro scale, multicellular forms might exploit new niches inaccessible to single cells, reshaping entire ecosystems.
A multi-level model can couple, for instance, a gene regulatory network (GRN) at the cellular level to population-level equations for cluster growth, culminating in ecosystem-level resource flux models. Changes in the GRN can lead to new morphological or cooperative phenotypes, which scale up to influence resource availability or predator–prey dynamics. Conversely, ecological conditions may select for or against certain cell-level traits. This nested approach mirrors a dialectical layering, where each level is both cause and consequence of processes at adjacent levels.
Some might argue that bridging multiple scales is overly complex, possibly losing clarity in the swirl of interactions. Yet the impetus for multi-scale modeling arises naturally when single-level approaches cannot explain phenomena like emergent morphological patterns, evolutionary transitions in individuality, or ecosystem engineering. Dialectical logic ensures that we treat these scales as interactive rather than strictly hierarchical. Empirical data — spanning, for example, transcriptomic analyses in developing organisms, population growth assays, and community-level biodiversity measures — can validate whether multi-scale feedback loops accurately capture observed evolutionary outcomes.
Together, these seven themes — Being and Becoming, Dialectical Natural Selection, Cooperation and Symbiosis, Horizontal Gene Transfer and Epigenetics, Niche Construction and Environmental Feedback, Chance and Necessity, and Cross-Scale Integration — offer a panoramic view of how dialectical logic clarifies the dynamic tensions that underlie evolutionary processes. Rather than seeing evolution as a one-dimensional path shaped by isolated forces, each theme highlights feedback loops, contradictory impulses, and emergent structures that reshape the very parameters of adaptation.
What unites these themes is an emphasis on recursive causation — the environment influences organisms, organisms modify the environment; cooperative relationships morph into exploitative ones and back again; random events precipitate deterministic cascades; and historical constraints define the present while generating novel futures. In each case, the formal methods discussed previously — dynamical systems, agent-based simulations, game-theoretic models, network analyses — can be tailored to capture the nuanced feedbacks and multi-level interactions at play. Meanwhile, empirical data (ranging from microbial consortia to large-scale ecological or phylogenetic studies) can be used to calibrate and test whether the predicted dialectical patterns indeed manifest in nature.
V: Empirical Validation and Testable Predictions
Moving from theoretical exposition and modeling to concrete, data-driven inquiry is essential for any robust scientific framework. A dialectical approach to evolutionary theory, with its emphasis on feedback loops and emergent contradictions, must demonstrate explanatory and predictive power in the empirical domain. This section examines how key dialectical principles — such as recursive organism–environment interactions, historical constraints, and multi-scale processes — can be empirically validated. It also outlines testable predictions and experimental/observational designs that researchers can adopt to assess whether evolution indeed proceeds via dialectical dynamics.
V.1 Empirical Case Studies Aligned with Models
A critical step in showing that a dialectical framework has real-world validity is to align formal models with empirical case studies, drawing data from lab experiments, field research, and comparative analyses. By carefully designing studies that capture feedback loops and historical contingencies, we can calibrate model parameters, evaluate predictive success, and refine theoretical assumptions.
V.1.1 Microbial Evolution Experiments
Microbes provide some of the most tractable systems for testing evolutionary hypotheses. They reproduce quickly, mutate readily, and can be subjected to precise environmental manipulations in the laboratory. From the perspective of dialectics, microbial populations are invaluable because they exhibit real-time feedback: as they consume resources or excrete waste products, they alter their microenvironment, which then shapes subsequent selection.
To test a dialectical model, one could set up multiple chemostat or batch-culture experiments where a microbial population evolves under well-controlled conditions. Measurements would include:
- Population size over time
- Resource levels (e.g., nutrient concentrations)
- Phenotypic traits (e.g., growth rate, metabolic enzyme profiles)
- Genomic or transcriptomic snapshots at intervals
Researchers would then compare the observed oscillations, stabilizing equilibria, or phase shifts in trait frequencies to a time-lagged differential equation or agent-based framework that encodes resource depletion, accumulation of byproducts, and emergent selection. If the model anticipates cyclical booms and busts or rapid evolutionary branching under specific parameter regimes — and these are observed in the lab — the system provides quantitative support for the proposed dialectical processes.
While microbes are relatively simple, it can still be difficult to distinguish purely negative frequency-dependent selection from more complex dialectical feedback. The environment must be sampled frequently enough (e.g., measuring local pH, toxin buildup) to confirm that organism-driven environmental changes are indeed driving the observed evolutionary patterns.
V.1.2 Long-Term Field Studies
Field studies of plants, animals, or microbes in their natural habitats capture multi-dimensional feedback mechanisms — ranging from niche construction to community-level interactions. Dialectical processes can be especially evident when the focal organisms actively modify key environmental parameters (e.g., nutrient cycles, soil structure, or habitat architecture), or when historical legacies (such as repeated disturbances) continue to shape population dynamics.
Consider a longitudinal study of a dam-building species like beavers or a root-nodule–forming legume. Over years or decades, researchers can track:
- Shifts in local ecology (e.g., water chemistry, vegetation composition)
- Population changes of the focal species and associated community members
- Trait evolution in response to the constructed or modified niche
Dialetical models might be invoked to predict the direction and speed of environment-driven selection. For instance, if beavers intensify dam-building, the standing water area might expand. Model predictions could be tested against observed population expansions in certain aquatic species, changes in predator–prey relationships, or morphological traits (like beaver tooth enamel thickness) that facilitate more frequent gnawing.
Field conditions are messy, with multiple confounding factors — weather patterns, invasive species, human disturbances — that may obscure or amplify the effects of niche construction. Researchers need robust statistical frameworks (e.g., structural equation modeling) to tease apart which feedback loops are most important and whether they align with a dialectical dynamic rather than simple unidirectional selection.
V.1.3 Comparative Genomic and Phylogenetic Studies
Modern genomics offers a powerful lens on historical contingencies and lateral genetic exchanges that might otherwise remain invisible. By comparing gene sequences, regulatory architectures, or epigenetic marks across related species, one can detect signatures of dialectical constraints (e.g., path-dependent vestiges) or transformations (e.g., horizontally acquired gene clusters prompting ecological shifts).
A typical approach involves constructing a phylogenetic tree of related species that differ markedly in some trait (e.g., antibiotic resistance in bacteria, flight capability in birds, or venom composition in reptiles). Researchers can:
- Identify shared or unique genetic elements
- Map traits onto the phylogeny
- Infer historical timescales of trait acquisition or functional gene replacement
Dialectical patterns might appear as punctuated changes in genes controlling environmental interactions (e.g., nitrification pathways in soil microbes) that align with historical events (like a niche shift) and lead to new evolutionary routes. If an environment-building trait appears, and subsequent lineages exploit that environment in new ways, this indicates a feedback loop consistent with dialectical predictions.
Comparative analyses are retrospective, so pinning down cause-and-effect relationships requires careful correlation with environmental history (e.g., paleoclimatic data, geological shifts). Moreover, gene transfer might appear sporadic rather than systematically driven by a feedback dynamic. Researchers can employ “ancestral state reconstruction” or co-phylogenetic methods to see if acquisitions of new genetic modules consistently coincide with environment or niche changes in a dialectical pattern.
V.2. Testable Predictions and Experimental Designs
Beyond aligning existing data with dialectical models, the framework calls for prospective predictions that can be experimentally or observationally tested. These predictions often revolve around how evolutionary trajectories diverge when feedback loops or contradictions are manipulated in a controlled manner.
V.2.1 Manipulating Feedback Loops in Controlled Ecosystems
If dialectical models are correct, then artificially enhancing or suppressing the organism–environment feedback should produce different evolutionary trajectories than “control” conditions where no such manipulation occurs.
Experimental Setup
- Microcosms or Mesocosms: Researchers can construct micro-ecosystems (e.g., soil or aquatic environments) with known species compositions.
- Feedback Manipulation: Introduce a mechanism (e.g., a mechanical filter, chemical neutralizer) that reduces the effect of the organisms on the environment, effectively breaking or dampening the feedback loop. Alternatively, amplify the feedback by adding resources that organisms specifically modify, intensifying their ability to shape local conditions.
- Measured Variables: Track population dynamics, trait distributions, resource states, and emergent structures (e.g., biofilm formation, nest building).
Predictions
- Damped Feedback: Without strong environment-modification, trait evolution might settle into simpler patterns (e.g., an equilibrium under classical selection).
- Amplified Feedback: Rapid oscillations, localized collapses, or the emergence of alternative stable states could appear, reflecting powerful dialectical tensions.
- Data Analysis: Statistical checks for cyclical or multi-stable trajectories can confirm the presence of dialectically patterned evolution.
Practical Considerations
Mesocosm experiments must be large or long enough to capture multi-generational processes. Researchers should also ensure that the manipulated feedback is indeed relevant to the focal trait(s). Setting up replicate treatments in parallel helps distinguish general patterns from chance anomalies.
V.2.2 Multi-Generational Tracking of Epigenetic Inheritance
Epigenetic modifications act as a dialectical bridging between environment and genetics: if an environmental stress triggers heritable modifications, the resulting phenotypes feed back to shape resource use or social structures. Over multiple generations, this can create emergent adaptive (or maladaptive) patterns inconsistent with strictly vertical, gene-centric models.
Experimental Setup
- Model Organisms: Plants, insects, or rodents known to exhibit epigenetic inheritance (e.g., paramutation in maize, maternal grooming effects in rodents).
- Stress Induction: Impose a controlled stressor (e.g., drought in plants, mild thermal stress in insects) that triggers an epigenetic response.
- Cross-Generational Observation: Over several generations, measure trait expression (physiological, morphological, or behavioral), epigenetic markers (methylation patterns, histone modifications), and changes in the environment (resource patterns, population density).
Predictions
- Short-Term Transmission: One or two generations might show direct epigenetic inheritance aligning with the induced stress.
- Longer-Term Dialectical Effects: If the phenotypic changes alter resource usage or social structures, subsequent generations may experience a different selection regime, possibly consolidating or reversing the epigenetic pattern. This cyclical dynamic is indicative of a dialectical feedback.
- Trait Reversibility or Ratcheting: Depending on how effectively the environment stabilizes new phenotypes, the system might show ephemeral shifts (reverting when stress ends) or lasting transformations that become partially coded into genetic or epigenetic architecture.
Practical Considerations
A challenge is distinguishing true transgenerational epigenetic inheritance from maternal effects or selection on cryptic genetic variation. Carefully controlled breeding designs (e.g., cross-fostering in animals, reciprocally transplanted seeds in plants) can help isolate epigenetic processes. Advanced molecular assays (bisulfite sequencing, histone ChIP-seq) are crucial to confirm that putative epigenetic markers coincide with phenotypic outcomes.
V.2.3 Adaptive Radiations Under Changing Environments
Dialectical models predict that innovations can restructure selection landscapes, causing adaptive radiations that differ from standard one-step expansions. If the environment co-evolves (through either niche construction or species-driven changes), we should see repeated cycles of morphological or genetic diversification.
Experimental/Observational Setup
- Experimental Evolution with Niche-Modification: In the lab, introduce a population of artificially “modular” organisms (e.g., digital organisms or engineered microbes) capable of drastically altering resource availability. Observe whether morphological or genetic diversification accelerates once a certain threshold of environmental modification is reached.
- Observational Studies in Natural Systems: Look for real-world “radiation pulses” associated with a key innovation, such as cichlid fish developing a novel feeding apparatus that transforms resource usage in a lake. Gather time-series data (fossil or morphological if possible) to see if repeated bursts of speciation occur following environmental reconfiguration.
Predictions
- Burst–Plateau Pattern: A new trait or combination of traits triggers an initial burst of diversification (due to newly available niches). After saturating those niches, a plateau or partial collapse might follow — unless secondary innovations arise, prompting further expansions.
- Frequency-Dependent Shifts: As one lineage occupies a niche, the environment changes in ways that open or close opportunities for other lineages. This dynamic system can be tracked by morphological or genetic means, revealing cyclical expansions or sporadic leaps.
Practical Considerations
Empirical data on adaptive radiations can be coarse-grained (e.g., fossil records with incomplete resolution). However, combining morphological analyses, ecological measurements, and population genetics can approximate whether expansions coincide with a species actively reshaping the local environment. In laboratory contexts, replicate lines serve as a check: if repeated experiments show similar cycles of innovation and stasis under carefully controlled conditions, that supports the notion that environment-shaping by the organisms triggers recurring dialectical waves of diversification.
V.2.4 Measuring Dialectical Complexity in Natural Communities
If a community is strongly governed by dialectical dynamics, we expect to see quantifiable indicators of interdependent feedback loops — rather than purely linear relationships — among species, resources, and abiotic factors.
Measurement Approach
Researchers can develop a “dialectical complexity index” (DCI) that aggregates:
- Network reciprocity: The extent to which changes in one node (e.g., a key species) have immediate and later-stage influences on multiple other nodes, which in turn feed back to the original node.
- Temporal layering: The presence of delayed or memory-based effects.
- Emergent transitions: Observed non-linear shifts in community composition in response to small environmental or demographic perturbations.
Data can be collected through long-term ecosystem monitoring projects — such as the dynamics in grasslands, coral reefs, or forest plots. Statistical tools (e.g., Granger causality tests, structural equation modeling, or partial least-squares path modeling) can disentangle which interactions are strongly reciprocal vs. those that appear one-directional. High DCI values would imply strong dialectical feedback, possibly visible as cyclical changes in species dominance or repeated transitions in vegetation structure.
Predictions
Systems with high DCI should exhibit:
- Resilience–Fragility Paradoxes: They may be extremely stable under certain conditions (because feedback loops self-correct small perturbations) but prone to sudden shifts once thresholds are crossed.
- Resource “Boom–Bust” Cycles: Coevolving consumer–resource or mutualistic networks that cannot be explained by standard predator–prey or logistic models.
- Non-trivial Legacy Effects: Disturbances (like fires or storms) produce consequences that manifest years or decades later, reflecting system memory.
Practical Considerations
Constructing such an index requires careful operational definitions of reciprocity, memory, and emergent transitions, as well as robust data on species interactions, resource dynamics, and environmental variables. Mixed methods — combining remote sensing (for large-scale changes), ground-based surveys (for biodiversity and population structure), and molecular analyses (for gene flow or epigenetic changes) — can increase confidence in identifying truly dialectical patterns.
V.3 Anticipating Critiques and Refinements
The empirical strategies outlined above are neither trivial nor guaranteed to yield neat, unambiguous results. Critiques may revolve around:
- Overfitting: Dialectical models with numerous parameters or feedback terms might appear flexible enough to “explain” almost any outcome.
- Ambiguity in Negative vs. Positive Tests: A lack of observed cyclical or reciprocal patterns might stem from suboptimal sampling intervals or experimental conditions, rather than a genuine absence of dialectical processes.
- Context-Dependence: Dialectical patterns could be more relevant in some contexts (e.g., strong niche-constructing species, intense epigenetic inheritance) and negligible in others (e.g., strictly gene-centric microbe populations in stable environments).
To mitigate these concerns, replication and comparative analysis across multiple systems are crucial. If multiple studies under different contexts and with varied focal species detect consistent dialectical signals — feedback-driven cycles, memory-dependent transitions, emergent radiations — then confidence grows that such processes are indeed widespread. Moreover, the dialectical perspective encourages iterative refinement: each empirical mismatch spurs a re-examination of which feedback loops are truly operative, whether unmodeled variables are at play, and how the timescales for memory or historical legacy might differ among species.
V.4 Methodological Considerations for Rigor and Transparency
Because dialetical research will often involve complex, multi-layered models and diverse empirical data, researchers must exercise methodological rigor at every stage:
- Calibration and Sensitivity Analysis: When aligning models to data, systematically vary parameter values to identify stable or chaotic regions in the model’s parameter space. Document any assumptions made about environmental carrying capacity, mutation rates, or resource regeneration.
- Cross-Validation: Whenever possible, separate the dataset into calibration vs. validation subsets. For example, use early-stage microbial data to tune model parameters, then predict mid- or late-stage population dynamics to see if the model genuinely foresees novel phenomena.
- Transparent Reporting: Provide full details on how memory terms, feedback intensities, or ecological interactions are parameterized. If certain data (e.g., resource levels) are only measured intermittently, report how interpolation or smoothing might affect detection of feedback loops.
- Interdisciplinary Collaboration: Dialectical studies often sit at the intersection of ecology, genetics, developmental biology, computational science, and philosophy. Engaging multiple specialists can prevent oversights, enhance data collection quality, and enable robust model building.
- Pre-Registration of Hypotheses: Particularly for new or controversial predictions (like cyclical transitions in epigenetic states across generations), pre-registering experimental protocols and hypotheses can bolster confidence that reported patterns are not merely post hoc interpretations.
V.5 Pathways for Further Empirical Exploration
Looking ahead, emergent research directions that could further validate the dialectical framework include:
- Synthetic Biology Platforms: Engineering microbial consortia or multicellular systems with tunable feedback loops, effectively creating “sandbox” environments where the dialetical interplay of traits and environment can be precisely manipulated and monitored.
- Genome Editing and CRISPR-based Tools: Introducing or disabling horizontal gene transfer capabilities (e.g., plasmid conjugation machinery) in microbial strains to observe how drastically it shifts evolutionary dynamics under environmental pressures.
- Global-Scale Observational Data: Leveraging big-data repositories in ecology or climate science to track large-scale patterns, such as how deforestation or agricultural practices (major niche constructions) restructure entire ecosystems, leading to unanticipated evolutionary shifts in local species.
- Comparative Evolutionary “Smart” Databases: Automated pipelines that continually incorporate new genomic and ecological data, searching for signatures of cyclical or historical feedback consistent with dialectical theory.
- Co-evolution of Technology and Theory: As machine learning methods become more sophisticated, they can help detect hidden reciprocities and emergent transitions in large datasets — potentially revealing dialectical patterns that simpler statistical methods miss.
Empirical validation and testability represent the linchpin that connects the lofty, integrative ambitions of a dialectical evolutionary framework with the day-to-day realities of data collection, experimental design, and statistical analysis. By focusing on how to detect feedback loops, when to recognize path dependence, and what emergent properties define a truly dialectical dynamic, researchers gain not only theoretical insight but also practical methodologies for advancing evolutionary science.
Each empirical strategy — whether harnessing microbial evolution experiments, field studies of niche-constructing organisms, comparative genomics, or specially designed manipulations — challenges the notion that evolution is solely about incremental changes filtered by a static environment. Instead, these approaches invite us to witness evolution as a dance of contradictory forces, historical legacies, and emergent novelties — a dance that can be quantified and predicted with proper models and carefully collected data.
VI: Iterative Feedback & Recursive Synthesis
Developing a comprehensive evolutionary theory that treats dialectical processes as central requires not just formulating models and collecting data, but also creating a meta-framework in which theory and evidence interact iteratively. The hallmark of dialectical thinking is that no single result is final; instead, knowledge evolves through recursive cycles that incorporate new insights, refine models, and generate further predictions. This section lays out how a dialectical approach can systematize the ongoing refinement of evolutionary theory, ensuring that conceptual and empirical endeavors continually inform each other in an organized, transparent, and self-correcting manner. Common criticisms — such as worries about circular reasoning, over-formalization, and defining dialectical complexity — are addressed to show how the dialectical method, properly implemented, mitigates these concerns rather than exacerbating them.
VI.1 A Meta-Framework for Continuous Refinement
VI.1.1 The Logic of Iterative Feedback
In a dialectical worldview, knowledge itself is subject to the same dynamics of tension, negation, and synthesis that characterize natural processes. This implies a circular or spiral logic for scientific inquiry: each stage of modeling is tested empirically; the resulting data reveal potential contradictions or gaps; these tensions prompt modifications in assumptions, parameters, or conceptual structure; and the cycle begins anew. The cycle never “concludes” in the sense of producing an unassailable final theory; instead, it deepens and expands in scope, incorporating more data and better approximating the complexities of evolution.
- Model Construction
- Objectives: Identify which dialectical tensions (e.g., environment–organism feedback, epigenetic memory) will be modeled.
- Assumptions & Parameters: Determine what level of detail is tractable — e.g., continuous vs. discrete variables, memory terms vs. real-time interactions, single-level vs. multi-level design.
2. Empirical Testing
- Data Collection: Gather relevant observations or experimental outcomes (microbial evolution, field ecology, comparative genomics).
- Model–Data Comparison: Calibrate parameters, check whether predicted trajectories match observed patterns, and look for discrepancies.
3. Contradictions & Refinements
- Identify Tensions: Where do the data conflict with model assumptions? Which phenomena remain unexplained?
- Refine or Revise: Adjust the model or incorporate new feedback loops and constraints. If the mismatch is fundamental, revisit conceptual premises (e.g., is the environment truly exogenous, or do we need a more nuanced feedback function?).
4. Synthesis & Further Questions
- Iterative Expansion: The refined model or conceptual framework now addresses previous contradictions.
- New Predictions: Because the theory has evolved, it can generate novel hypotheses to be tested in the next cycle.
VI.2 Addressing Potential Criticisms & Avoiding Circular Reasoning
Despite its virtues, a dialectical methodology can invite skepticism if it appears to rationalize every outcome as part of an ever-shifting feedback loop. Critics may worry that a “dialectical lens” could become all-encompassing — dismissing anomalies as mere contradictions waiting to be synthesized, thus never risking genuine falsification. Below, common criticisms are articulated and robust responses grounded in methodological rigor are proposed.
VI.2.1 “Dialectical Complexity” as a Vague, Catch-All Concept
Critique
Some might argue that labeling phenomena “dialectical” might be a way of saying “it’s complicated,” offering no real predictive or explanatory power beyond standard ecological or evolutionary models.
Response:
- Operational Definitions: When referencing “dialectical complexity,” researchers must specify quantifiable attributes — such as feedback intensity, path-dependence indices, or reciprocal interaction coefficients — that can be measured.
- Comparative Models: Parallel runs of a dialectical vs. a traditional model can highlight if incorporating reciprocal environment–organism modifications (for example) significantly improves predictive accuracy. If it does, then “dialectical complexity” is not just rhetorical flourish.
- Empirical Grounding: Indices or metrics can be validated against real data, ensuring that “dialectical complexity” is tied to observable phenomena (e.g., cyclical resource–population fluctuations, sudden morphological shifts induced by historical constraints, etc.).
VI.2.2. Danger of Over-Formalization
Critique
Another line of criticism holds that attempting to mathematically encode every dialectical feedback results in unmanageable models loaded with parameters and assumptions, making them susceptible to overfitting and losing interpretive clarity.
Response:
- Iterative Parsimony: Start with simpler core feedback loops — such as environment–trait couplings — and only add complexity if repeated empirical mismatches necessitate it. Document each addition’s justification (e.g., epigenetic inheritance turned out to be crucial for capturing multi-generational trait shifts).
- Modular Design: Instead of building one giant model, use a modular approach where separate submodels handle distinct layers (e.g., resource dynamics, population genetics, epigenetic regulation). Each submodel can be tested independently before being integrated.
- Rigorous Model Selection: Employ statistical or information-theoretic metrics (AIC, BIC) to compare the explanatory power of simpler vs. more complex models. Over-parameterized frameworks can be penalized, encouraging a balance between complexity and parsimony.
VI.2.3. Risk of Circular Reasoning
Critique
A persistent worry is that dialectical logic might attribute every empirical pattern to “contradictions” or “feedback loops,” rendering the theory unfalsifiable. Contradictions become signals of progress instead of potential refutations.
Response:
- Hypothesis-Driven Testing: Dialectical theorists should propose clear, falsifiable predictions — for example, “Increasing the organism–environment feedback parameter beyond a certain threshold will produce cyclical population dynamics in a lab microcosm.” If no cycles appear in replicate experiments, that portion of the model is falsified, prompting either an alternative explanation or a reversion to simpler assumptions.
- Independent Validation: Empirical checks must involve variables or data not used in the original model calibration. This ensures that “explanations” do not simply rationalize anomalies post hoc but genuinely predict new observations.
- Transparent Contradictions: Document contradictions explicitly rather than interpreting them as automatically beneficial. The shift from contradiction to synthesis only holds if the synthesis yields new testable predictions or improved model consistency. Contradictions that cannot be resolved or lead to endless parameter fiddling should be recognized as serious theoretical failures.
VI.3. Strategies for Minimizing Methodological Pitfalls
To ensure that iterative feedback and recursive synthesis do not devolve into a spiral of subjective theory-tweaking, researchers can adopt methodological safeguards aligned with best practices in computational and empirical sciences.
VI.3.1 Structured “Checkpoints”
Regularly scheduled review points — perhaps at set intervals in a long-term research project — allow the team to systematically compare model outputs with new empirical data. If persistent mismatches remain, the group must deliberate whether to refine the model or consider discarding certain dialectical assumptions. Documenting these discussions in lab notebooks or shared archives preserves an audit trail of scientific reasoning, preventing silent or arbitrary model changes.
VI.3.2 Peer Collaboration and Interdisciplinary Panels
Because dialectical approaches often span multiple scales (from molecular to macroevolutionary) and weave together philosophical, computational, and empirical strands, it is vital to involve diverse expertise. Interdisciplinary panels, including population geneticists, ecologists, philosophers of science, and statisticians, can:
- Evaluate whether a proposed revision genuinely improves clarity or simply multiplies parameters.
- Question the necessity of certain dialectical features if simpler alternatives suffice.
- Offer external validation or skepticism, forcing consistent logic and reducing groupthink.
VI.3.3 Encouraging Reproducibility and Data Sharing
To test dialectical models robustly, data on trait distributions, resource usage, and environment–organism interactions should be openly shared, allowing independent groups to replicate analyses. Reproducibility is especially important when calibrating non-linear or agent-based models, which can be sensitive to small changes in initial conditions. By publishing code, parameter files, and full details of computational runs, researchers invite scrutiny that weeds out hidden circularities. This transparent culture also fosters collaborative synergy, as different labs might compare how their respective systems align with or challenge a given dialectical construct.
VI.4 The Recursive Nature of Scientific Knowledge
Beyond method and protocol, the spirit of a dialectical framework sees evolutionary theory itself as evolving through recapitulated cycles of contradiction and synthesis. Historical examples abound:
- Darwin vs. Mendelian Genetics
- Darwin’s emphasis on gradual variation clashed with discrete Mendelian inheritance, prompting the eventual Modern Synthesis.
- This “contradiction” was not resolved by discarding one side but by forging a new conceptual architecture that absorbed both.
2. The Modern Synthesis vs. Epigenetics, Niche Construction, HGT
- The Modern Synthesis explained a wide array of phenomena but left out emergent complexities.
- New lines of evidence introduced contradictions — vertical transmission alone was insufficient, the environment was not purely external — leading to expansions of evolutionary theory.
3. Current Debates and the Extended Evolutionary Synthesis
- Contemporary calls for an Extended Synthesis revolve around the tension between a gene-centric view of inheritance and the manifold ways organisms actively shape their own evolutionary trajectories.
- A dialectical perspective can mediate these debates, turning them into iterative refinements rather than polarized standoffs.
Crucially, each resolution is partial. Further data or newly revealed phenomena spark fresh cycles of refinement. By explicitly recognizing this recursive intellectual process, evolutionary biologists and philosophers can plan for systematic integration rather than ad hoc patchworks. A dialectical lens thus encourages meta-awareness of how science progresses: it is never a linear accumulation of facts but a dynamic interplay of contradictory findings that, when properly engaged, yield deeper theoretical insights.
In sum, a dialectical view of evolutionary theory demands a corresponding methodology for scientific inquiry: one that embraces iterative refinement, systematically grapples with contradictions, and synthesizes new insights into emergent models. Rather than being a license for endless speculation or untestable claims, this framework — if rigorously applied — functions as a self-correcting cycle that mirrors the very processes it seeks to explain. By structuring protocols for model revision, encouraging collaboration across disciplines, and insisting on clear, falsifiable predictions, the dialectical approach can maintain scientific integrity while opening new vistas of evolutionary understanding.
The payoff is that evolutionary science, practiced dialectically, becomes especially adept at addressing complex phenomena — from epigenetics to multi-level selection and dynamic niche construction — that elude simpler models. Over time, these recursive cycles may yield an increasingly nuanced picture of how life diversifies and innovates by constantly reshaping — and being reshaped by — its environment and internal constraints. In the final analysis, iterative feedback and recursive synthesis stand as core principles not just for how evolution operates in nature, but for how we, as scientists and philosophers, can keep our understanding of evolution alive, adaptive, and open to novel syntheses.
VII: Conclusion and Future Directions
Dialectical thinking injects a distinctive vitality into evolutionary theory: it invites us to recognize that each stage of biological change emerges through the interlocking of opposing forces, cyclical feedbacks, and emergent novelties that can reshape previously stable contexts.
Dialectical evolution has a dual commitment: on the one hand, it relies on formal mathematical and computational methods to capture feedback loops with precision; on the other, it draws on narrative, historical, and philosophical insights to situate these loops within broader contexts of being, becoming, and emergent complexity.
The dialectical view goes beyond typical “extended” or “integrative” expansions of evolutionary theory by underscoring the processual and tensional nature of scientific understanding itself. In philosophical terms, it draws attention to the idea that knowledge evolves through confrontation with anomalies, and that conceptual change is often triggered when established models meet phenomena they cannot explain without modification. For evolutionary science, this means:
- Revisiting Reductionism: Traditional reductionism tries to isolate single causes (genes, selection coefficients, etc.). Dialectical frameworks instead see these as nodes in broader webs of reciprocal influence. Methodologically, this means stepping away from single-cause explanations and instead learn to expect interplay among numerous factors — genes, epigenetic states, social behaviors, environmental manipulations — and to interpret that interplay as more than just an additive sum.
- Recasting Teleology and Directionality: Standard evolutionary theory disavows teleology, yet many accounts still subtly rely on adaptationist stories that seem to frame evolution as “problem-solving.” Dialectics replaces such teleological notions with feedback loops that yield emergent directionalities: not because organisms “strive” toward goals, but because historically contingent traits restructure the environment in ways that bias future evolutionary paths. This line of thought encourages reevaluations of how the discipline narrates evolutionary progress or complexity, preferring to see them as consequences of recursively stabilized innovations rather than purposeful endpoints.
- New Criteria for Theoretical Progress: If dialectics highlights the synergy of contradictions, then theoretical progress in evolutionary biology can be measured by how effectively new frameworks incorporate — and make testable predictions from — previously irreconcilable data or conflicting models. This approach stands apart from “consilience” as typically understood, focusing on productive tensions that drive the field forward, rather than neat assimilation into a single grand theory.
- Complexity Science: with its emphasis on self-organization and multi-level emergent patterns, resonates well with dialectical evolution. Joint workshops could bring together complexity theorists, computational biologists, and evolutionary ecologists to refine modeling frameworks that explicitly encode historical constraints and cyclical organism–environment dynamics. Such collaborations might lead to the identification of universal “dialectical signatures” across fields — ranging from the evolutionary transitions in individuality to the co-evolution of technology and culture in human societies.
VII.1 Bridging Societal and Ethical Dimensions
Although the main thrust of a dialectical evolutionary theory is scientific and philosophical, it can have implications for broader societal or ethical issues. Evolutionary ideas have historically influenced how people view cooperation, conflict, resource management, and even human cultural evolution. Dialectical approaches may open up fresh perspectives in at least two arenas:
- Ecological Management and Conservation
- Conservation efforts often rely on static or linear assumptions about ecosystems. A dialectical stance suggests that species do not merely occupy habitats; they engineer them, and new feedback loops can amplify or mitigate environmental stress. A reforestation project that acknowledges how certain tree species alter soil composition and microclimates might plan for subsequent waves of species that either thrive in the new conditions or become detrimental if the feedback is not managed.
- By modeling potential feedback loops, conservation biologists can anticipate emergent outcomes — like invasive species that exploit newly engineered niches — and design adaptive management strategies.
2. Societal Analogies and Human Cultural Evolution
- Human societies, replete with technologies, social norms, and institutions, also exhibit patterns of niche construction, cooperation, conflict, and path dependence. While anthropologists and social scientists may prefer caution in drawing direct analogies, dialectical frameworks can stimulate cross-disciplinary dialogues on how feedback loops between technology, environment, and cultural norms shape human historical trajectories.
- Epigenetic research on stress and behavior in human communities can also spark reflection on how dialectical processes — internal contradictions between inherited tendencies and new cultural contexts — drive transformations in social norms or institutions.
VII.2 The Perspective of Dialectical Evolution
Ultimately, the impetus for a dialectical approach arises from the realization that evolution is rarely (if ever) a single-threaded process. Across timescales — whether it is the ephemeral everyday life of microbes or the geologic spans of macroevolution — organisms respond to, and actively reshape, an environment that is itself an evolving tapestry of constraints, resources, and cohabitants. Dialectics underscores that each evolutionary gain can harbor internal contradictions, each success can sow the seeds of future challenges, and every emergent structure can become the foundation for further transformations.
That perspective resonates with broader scientific traditions that see nature not as a static set of laws but as a dynamic interplay of forces. It dovetails with philosophical traditions that insist knowledge grows through contradiction, and with ecological and evolutionary traditions that highlight non-linear, contingency-laden pathways. By forging explicit methodological ties — through advanced models and well-designed experiments — dialectical evolution can shed light on some of biology’s most persistent puzzles: how major innovations arise, how lineages break free of apparently constraining legacies, how cooperation co-exists with conflict, and why certain evolutionary trends appear cyclical or punctuated rather than strictly incremental.
In conclusion, the dialectical approach to evolution is not a final statement on how life changes but a method of inquiry that thrives on tension, interplay, and the generative power of feedback loops. By formalizing these principles and grounding them in robust data, evolutionary theorists can better illuminate the fascinating tapestry of adaptation, novelty, and constraint that characterizes the living world. The future of this enterprise hinges on continuing to push conceptual and methodological frontiers — always ready to transform each newly uncovered contradiction into a gateway for deeper understanding.
Peter Robinson, 4 February 2025.
